Author: Dr. Inna Yakovleva
Abstracts:
Introduction: Impact of Connective Tissue Disorders. Connective tissue pathology is a base of formation chronic diseases in children and is associated with changes in certain levels of different types of collagen and an expressed immunodeficiency. Many connective tissue disorders (CTD) carry high risks for life-threatening heart and blood vessel conditions. The gastrointestinal tract, as it contains a lot of collagen, may also be impacted by the subsequent development of inflammatory-destructive damages.
This study aimed to evaluate the significance of collagen level content of the gastroduodenal tract in the formation of peculiarities of gastroduodenal pathology in adolescents.
Materials and methods: Inflammatory-destructive diseases of the gastroduodenal tract were studied in 155 adolescents 11 to 18 years of age. The traits of the CTD, including the Marfan syndrome, were determined relying on the Ghent criteria. CTD were revealed in 90 adolescents. 65 peers without CTD were included in the comparison group. Collagens content in the lamina propria of gastric-duodenum mucosa was evaluated by immunohistochemistry using monoclonal antibodies of collagen 1, 3, 4, 5 types. Biopsy samples were studied in the luminescent microscope using light filters. A complex evaluation of connective tissue parameters was carried out using the system correlation analysis.
Results: Gastrointestinal involvement against the background of the CTD is characterized primarily by motility disorders, including gastroesophageal reflux disease, and reflux-gastritis (77%; comparison group – 29%, p<0,001) and a reduced level of interstitial collagens. The average immunofluorescence intensity of collagen types 1 and 3 in patients with CTD was significantly lower (p<0,01). The connective tissue matrix of the mucosa is characterized by the structural transformation of collagen fibrils (wrong orientation, focal sclerosis, immaturity). It is accompanied by a decrease in mucosa functional ability with the development of the valve-sphincter failure. The form of mucosal lesions is chronic non-atrophic gastritis with simultaneous inflammation in the antral and fundal parts. Duodenal ulcer was identified rarely (10,0%; comparison group – 33,8%; p<0,035), and it was accompanied by the detection of Helicobacter pylori.
Conclusion: Thus, CTD in adolescents is characterized by a low level of interstitial collagens in gastric-duodenal mucosa, and accompanied by structural transformation of collagen fibrils, which is the basis of the formation of motility disorders with the subsequent development of gastritis and esophagitis against the background of reflux. The work provides grounds for employing rehabilitation measures connected with the prevention of reflux disease and the development of destructive lesions in children and adolescents with CTD.
Keywords
children and adolescents, gastroduodenal pathology, connective tissue dysplasia, collagen, reflux
Abbreviations
connective tissue (CT), connective tissue dysplasia (CTD), fluorescein isothiocyanate (FITC), gastrointestinal tract (GIT), gastroesophageal (GER) and duodenogastric refluxes (DGR), esophagogastroduodenoscopy (EGDS), monoclonal antibodies (MCA)
Full article
Introduction
Nowadays, the attention of scientists has been paid to the group of diseases related to the connective tissue (CT) congenital pathology that is characterized by its reduced strength, various functional disorders with a deficiency of components of the connective tissue and as a consequence, structural and functional anomalies of various organs and systems. Such anomalies of tissue structure are commonly known as connective tissue dysplasia (CTD) [1-3].
Connective tissue dysplasia is classified into two groups. The first group includes differentiated dysplasia with a known gene defect of a certain type of inheritance and with a clear clinical picture (Marfan, Ehlers-Danlos syndromes, osteogenesis imperfecta, etc.). These diseases are classified as hereditary collagen diseases – collagenopathies [4]. The second group consists of undifferentiated CTD, which is most common in pediatric practice. Undifferentiated dysplasia is a genetically heterogeneous pathology caused by changes in the genome due to multiple intrauterine effects on the fetus. Genetic pathology in undifferentiated CTD remains unidentified. The main characteristic of these dysplastic manifestations is a wide range of clinical changes without a definite clear clinical picture. Assignment of patients to the undifferentiated CTD group is possible if its manifestations are not specific to such connective tissue diseases as rheumatoid arthritis, scleroderma, systemic vasculitis, dermatomyositis, systemic lupus erythematosus [5, 6,7].
The biochemical marker that most often occurs in patients with congenital CT diseases is a change in ratios of different types of collagens. The type III collagen content increases, the quantity of “thick” collagen fibers decreases, and the quantity of “thin” collagen fibers, the ground substance, elastin, and fibrocyte content in reticular stroma increases. The structural transformations of collagen fibrils lead to disorders of tissues and organs that occur in polymorphic organ dysfunction [8, 9,10].
The gastrointestinal tract (GIT) as one of the organs that are richest in collagen is certainly involved in pathological processes against the background of CTD. Frequent detection of signs of connective tissue dysplasia in children with gastroenterological diseases (from 30 to 72%) and, conversely, the high incidence of gastrointestinal tract pathology against the background of this syndrome (57–88%) prove their relationship [11, 12, 13]. Dysplastic changes in the gastrointestinal tract include ptosis of abdominal organs, insufficiency of the cardia, diaphragmatic hernia, anomalies of shape and structure of esophagus, stomach, duodenum, microdiverticulosis of the intestinal tract, disorders of gastric und duodenal secretions, organ peristalsis as well as gallbladder anomalies. In this case, the presence of CTD syndrome causes certain features of metabolism, adaptation, and existence of the organism in conditions of defective collagen. [11, 12]. Stable and expressed disorders of intestinal tract biocenosis, damaged duodenal vessels with their hyalinosis, increased proteinase activity, and presence of collagen and elastin antibodies – all these factors demonstrate complex lesion mechanisms of GIT damage against the background of CTD. It has been proven that the symptoms of connective tissue dysplasia are more common in schoolchildren with chronic gastroduodenitis [13].
As a rule, the gastroenterologists identify the chronic polymorphic GIT pathology in the patients with the CTD syndromes: esophagitis, chronic gastroduodenitis, gastroesophageal (GER) and duodenogastric refluxes (DGR), bulbitis, dyspancreatism, cholecystitis or gallbladder dyskinesia, colitis and dysbacteriosis against the background of their developmental anomalies [14, 15, 16]. Summing up, the morphological ground of the CTD is the reduced content of specific collagen types or their impaired ratio that leads to the reduced CT strength of many organs and systems. These have been confirmed by histologic studies of the CT-rich structures – heart valves, vessels, and skin in CTD patients. However, no data on the CT state of the gastroduodenal mucosa with the study of the content of the main collagen types in children and adolescents have been found in the available literature.
The present study aimed to evaluate the impact of connective tissue disorders and the significance of collagen level content in the gastric and duodenal mucosa on the formation of peculiarities of gastroduodenal pathology in children and adolescents and improve criteria for the diagnosis in order to prevent said pathology at early stages.
- Materials and Methods
Inflammatory-destructive diseases of the gastroduodenal tract were studied in 155 children 11 to 18 years of age. The features of CTD were revealed in 90 adolescents (52 boys and 38 girls). Another 65 peers (38 boys and 27 girls) without CTD were included in the comparison group. The traits of the CTD (small structural abnormalities on the part of internal organs, hypermobility of joints, deformations of the spine and chest, skin, vascular and muscular anomalies), including the Marfan syndrome and Ehlers-Danlos syndrome, were determined relying on the Ghent criteria [17].
The diagnosis of chronic gastroduodenal pathology has been verified using the anamnestic, clinical, and laboratory data, as well as esophagogastroduodenoscopy (EGDS) with target biopsy. Endoscopic examinations were performed according to the generally accepted method. The state of the lumen, the number, and nature of gastric contents, condition of folds, the presence of hyperemia, edema, mucosa defects such as ulcer and erosion, gastroesophageal and duodenum-gastric refluxes were taken into account. Histological diagnosis of gastritis was established on the basis of a modified Sydney system [ 18]. The morphometric study of samples was performed on a microscope “Olympus BX-41” followed by microphotography.
The biopsy material was taken from the stomach (antrum, body of stomach, pyloris) and duodenum. The biopsy samples were fixed in 10,0% neutral formalin solution, dehydrated in alcohols, and embedded in paraffin. The sections 4-5 microns thick were stained with hematoxylin and eosin for a general assessment of the test tissue and morphometric study. Weigert`s resorcin-fuchsin staining, picrofuchsin solution according to Van Gieson and Mallory was used to identify and differentiate the connective tissue structures. Glycosaminoglycan complex was studied by staining with alcian blue according to Hotchkiss-McManus. To identify and assess the intensity of Helicobacter pylori colonization (Hp), the tissue specimens were subject to Romanowsky-Giemsa stain.
Collagens content in the lamina propria of gastric-duodenum mucosa was evaluated by immunohistochemistry using monoclonal antibodies (MCA) of collagen 1, 3, 4, 5 types (Novocastra Laboratories Ltd., UK) using the Coombs technique according to Brosman [19]. Biopsy samples were studied in the luminescent microscope using light filters FS-1-2, SZS-24, BS-8-2, and UFS-63.
The F(ab)-2-fragments of rabbit antibodies against the mouse immunoglobulins, which are with Fluorescein isothiocyanate (FITC) tagged, were used as a luminescent tag. The intensity of collagen luminescence was determined using the microfluorometer with FEU-35 and expressed in relative units that were equal to the current flowing through the measuring device that was expressed in microampere.
The statistical analysis was conducted with the use of variation statistics techniques. The reliability of divergences between the indicators was determined using the Fisher’s test, the χ 2-test, and Wilcoxon-Mann-Whitney test. Statistical significance was set at p < 0.05 and the 95% confidence intervals (CI) p < 0.05 was considered to indicate statistical significance. A complex evaluation of connective tissue parameters was carried out using the system correlation analysis.
- Results and Discussion
Signs of CTD and phenotypic features were detected with different frequencies and in different combinations in children with symptoms of gastroduodenal diseases, such as abdominal pain¸ heartburn, belch, decreased appetite, and nausea. These were craniocephalic, cartilage, skeletal abnormalities, as well as vascular, cutaneous manifestations, and also anomalies of internal organs. Of particular importance were the following signs – skeletal features such as thorax deformation, scoliosis, kyphosis (84.4%), Gothic palate (75.6%), joint hypermobility (73.3%), skin fragility and hyperextensibility (50.0%), sandal-shaped slit on the feet (50.0%), dysplasia of teeth – impaired tooth growth and enamel formation (45.6%). Small structural abnormalities of the heart (76.7%) were mainly represented by mitral valve prolapse (76.7%), aortic root dilation (23.3%), and abnormally placed chords or heartstrings (64.4%). Phenotypic features of CTD in the urinary and hepatobiliary systems were manifested in the form of enlargement of the pelvic system of the kidneys (38.9%), its branched type of structure (31.1%), nephroptosis (4.4%), shape anomalies or deformed gallbladder (40.0%) and constriction of the gallbladder and bile ductus (6.7%).
The analysis of the structure of the gastroduodenal pathology in adolescents with CTD showed that chronic gastroduodenitis made up the majority (73.3%), the chronic gastritis was the rare case (5.6%) that was not different from the indicators of the patients without the CTD signs. The duodenal ulcer was identified only in 5.6% of patients with CTD against 16.9% in the comparison group (p<0.01). The important role in the structure of the upper GIT pathology on the background CTD plays the gastroesophageal reflux disease with inflammatory and destructive changes in the esophagus (17.8 against 1.5% in the comparison group, p<0.001).
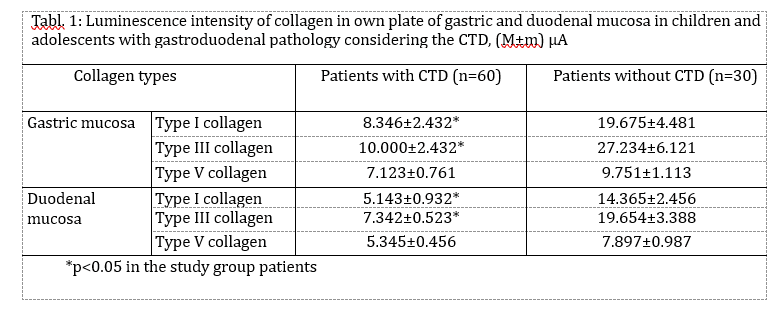
The endoscopic picture of gastric mucosa in adolescents of both groups was characterized by the presence in most cases of erythematous gastropathy (57.8% and 50.8%, respectively by groups) and mucosal hypertrophy (33.3% and 36.9%). Erosions in the antrum of the stomach (2,2% vs 10,2%, p<0,05) are revealed significantly more often in the comparison group. In particular cases, there occurred subatrophic (2.2% and 3.1%) and combined gastropathy (3.3% and 1.5%). (Fig.1).
The duodenal mucosa also showed erythematous duodenopathy (76.7 and 67.7%). The intensive erosion processes in the duodenal bulb were characteristic, but more frequently it was reliable in the group without CTD (6.7% against 20.0, p<0.01). Duodenal ulcer was identified rarely (10,0%; comparison group – 33,8%; p<0.05), and it was accompanied by the detection of Helicobacter pylori.
In the group of patients with CTD during Romanowski-Giemsa staining, HP bacteria in the antrum were visualized in 40.0% of cases and in 20.0% of cases – in the fundus of the stomach. In the comparison group, similar indicators were significantly higher and amounted to 83.3% and 33.3%, respectively. This suggests that the frequency of HP-associated forms of gastritis in adolescents with CTD is significantly lower (p <0.001).
The obtained results of endoscopic investigations showed that the patients with CTD had the refluxes (GER, DGR, and their combination) in 76.7% of cases that were likely to prevail their frequency in the group without dysplasia – 29.2% (p<0.001). The DGR was registered most frequently (76.7 and 27.7%, respectively, p<0.001), and the combination of the GER and DGR occurred only in the main group (14.4%, p<0.001). The isolated GER was registered in 3 patients with CTD (4.4%) and in 1 adolescent (1.5%) in the comparison group. It was established that the DGR in the main group was most frequently associated with superficial (60.6 and 50.8%) and hypertrophic gastritis (36.8 and 38.9%).
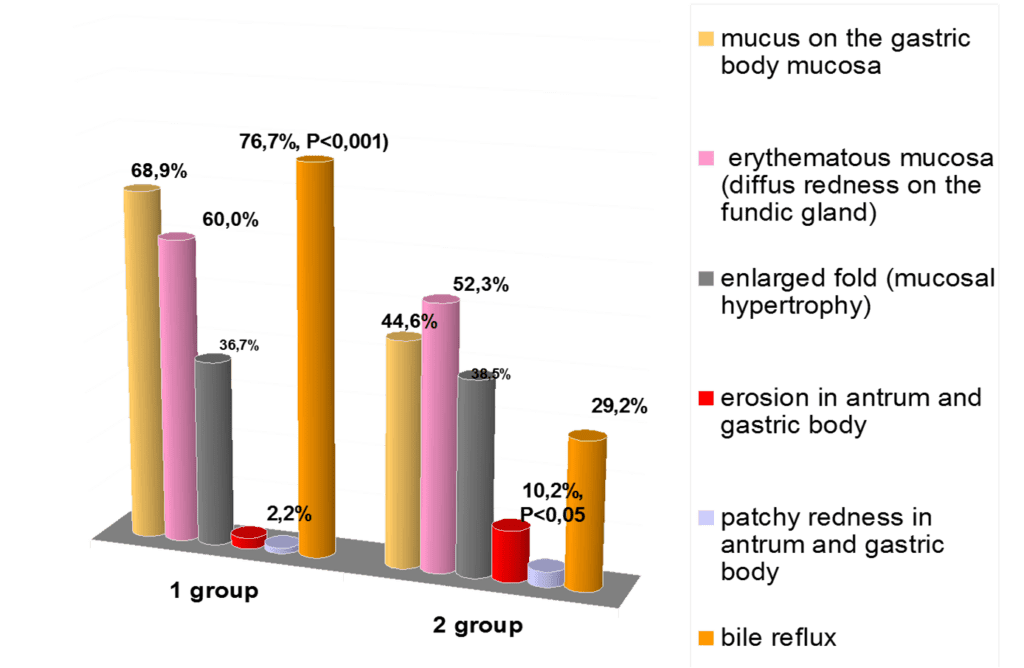
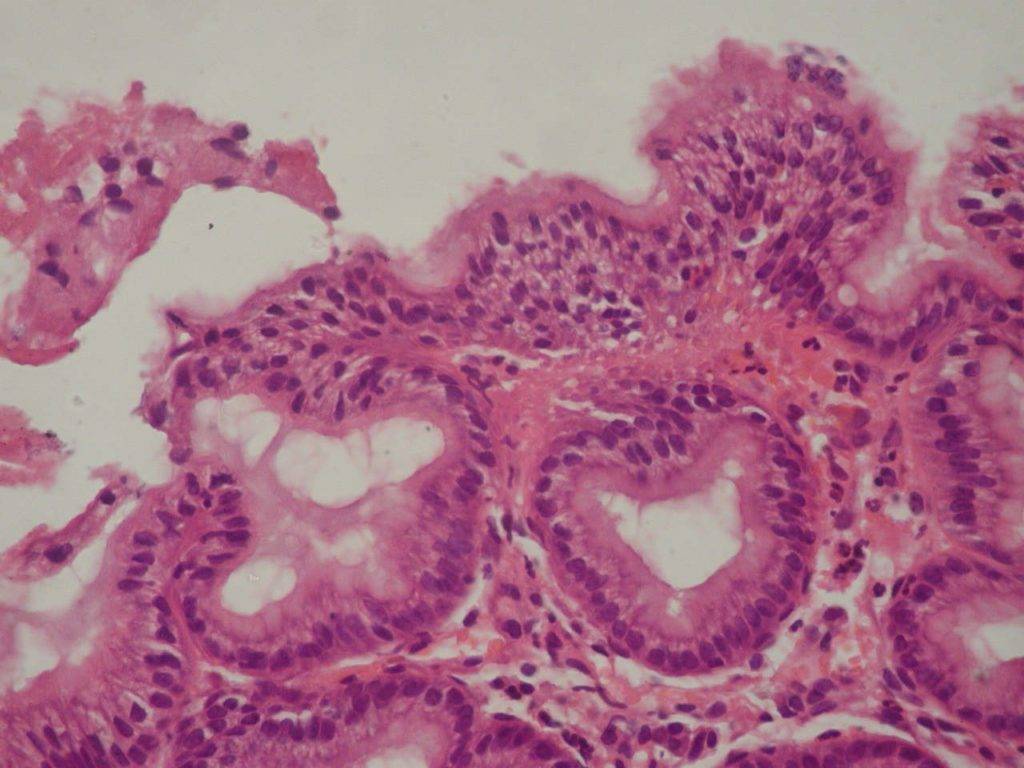
Foveolar hyperplasia of the antrum mucosa, which has been revealed in 60,0% of cases on the background of CTD (p<0,001), is a histopathological manifestation of reactive gastritis due to duodenal (bile) reflux. (Fig.2)
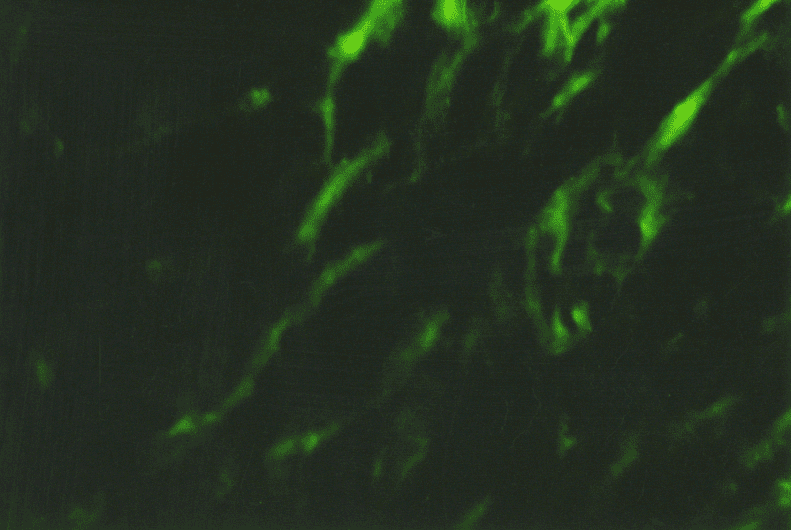
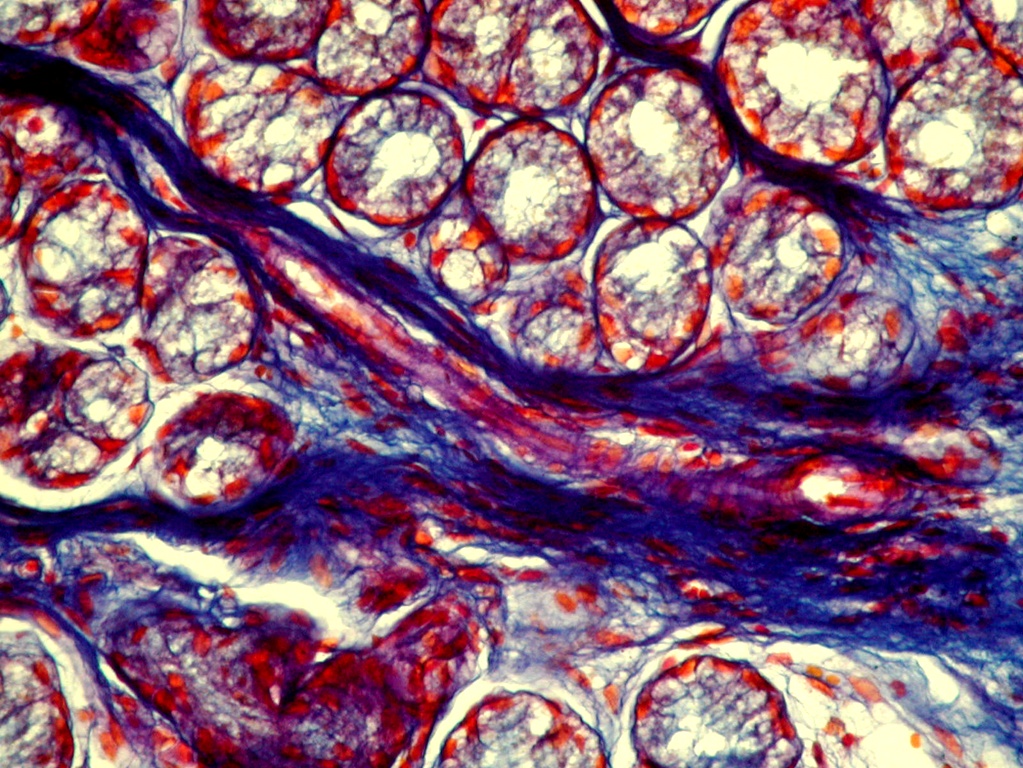
The immunohistochemical study of different collagen types made it possible to identify not only the localization of the main collagens in the own mucosa plate but also to determine the intensity of collagen formation in the CT fibers structures. Thus, in collagen fibers of lamina propria of the gastric and duodenum mucosa in both group patients the type III collagen, the known young interstitial collagen being the strengthening component of the walls of hollow organs, including the vessels and intestinal tract, prevailed. This collagen occurred in the form of luminescence of non-uniform local intensity (Fig. 3). The average intensity of luminescence of the type III collagen in the patients with CTD was likely to be lower than the indicator in the comparison group (p<0.05) (Table. 1).
The more mature interstitial type I collagen occurred in the form of linear luminescence of moderate intensity (Fig. 4). The average intensity of immunofluorescence of the type I collagen in adolescents with CTD also was likely lower than in the comparison group (p<0.05).
Except for the indicated two main interstitial collagens (I and III), the type V collagen was identified which was also the normal component of the interstitial tissue. The patients with CTD showed only the trend of a decreased degree of immunofluorescence of the specimens treated with MCA of the type V collagen (Table. 1). This collagen occurred in the form of local luminescence of non-uniform intensity: there were the areas where the luminescence was considered as bright as the areas with low luminescence.
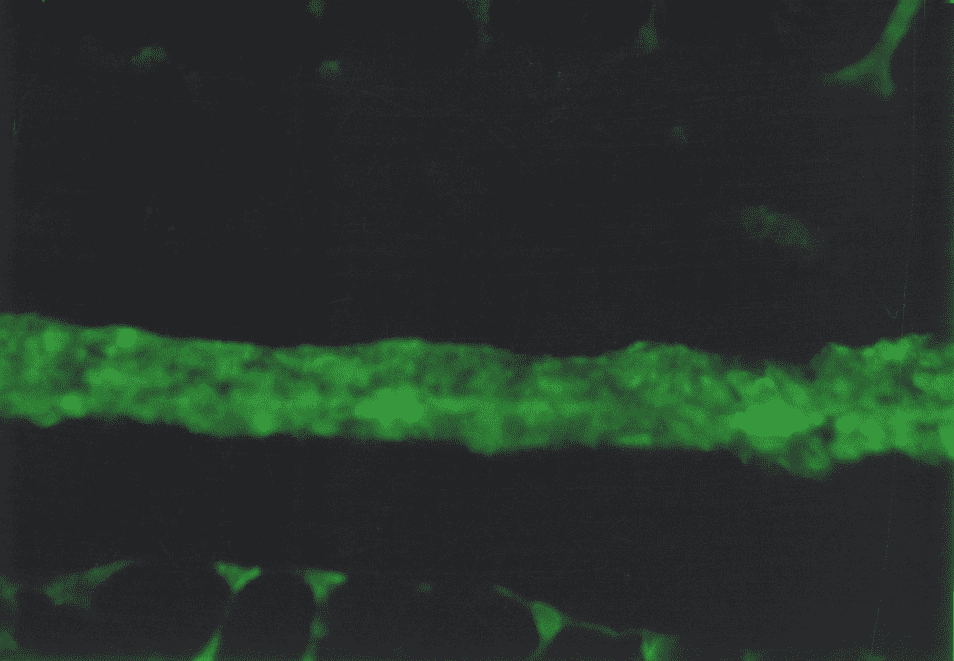
The immunohistochemistry study found the type IV collagen in basal subepithelial membranes of the gastric mucosa. The non-uniform luminescence of this collagen was observed. In the points of basal membrane thickening that correspond to the zones of colonization Hp, the areas of bright luminescence were found, and in the points of basal membrane peeling, the areas of low and moderate luminescence were found (Fig. 5).
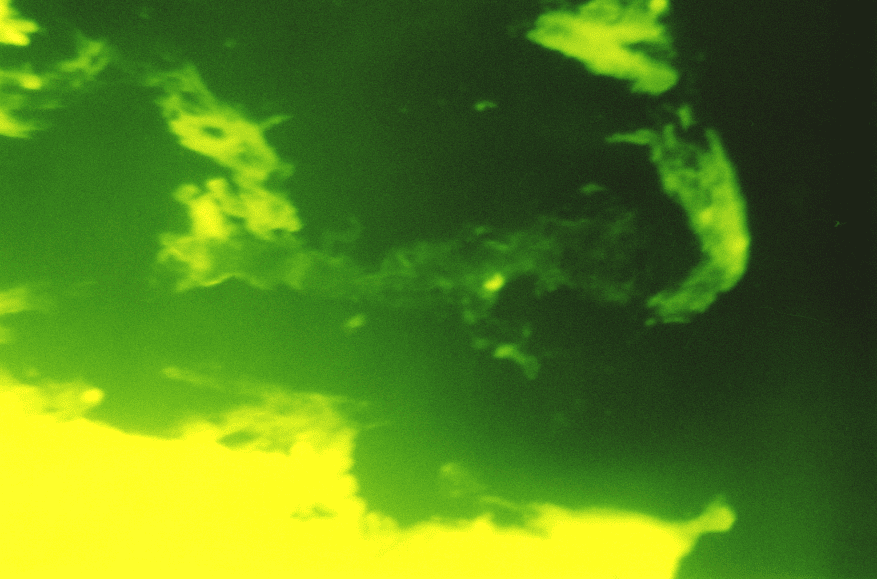
Under the subepithelial basal membrane, there was a dense network of reticular fibers with the type III collagen in the form of linear low-intensity luminescence as a component. In the zones of coarsened collagen fibers, the patients of the main group showed a considerable prevalence of type I collagen while the type III collagen was specified in the form of low and moderate-intensity luminescence. Also, there were the areas with bright luminescence and fuzzy structure of both collagens in the connective tissue component of the duodenum, it is not excluded that these were the destruction zones. (Fig.6)
In the stroma of the gastric mucosa of the main group paitients during Van Gizon staining, fibrous structures were found, which were characterized by uneven intensity of staining and distribution. At the same time, weakly fuchsinophilic fibers with foci of picrinophilia were often found, which reflects dystrophic and degenerative changes in the fibers, and fibers with incorrect orientation of fibrils were also detected.
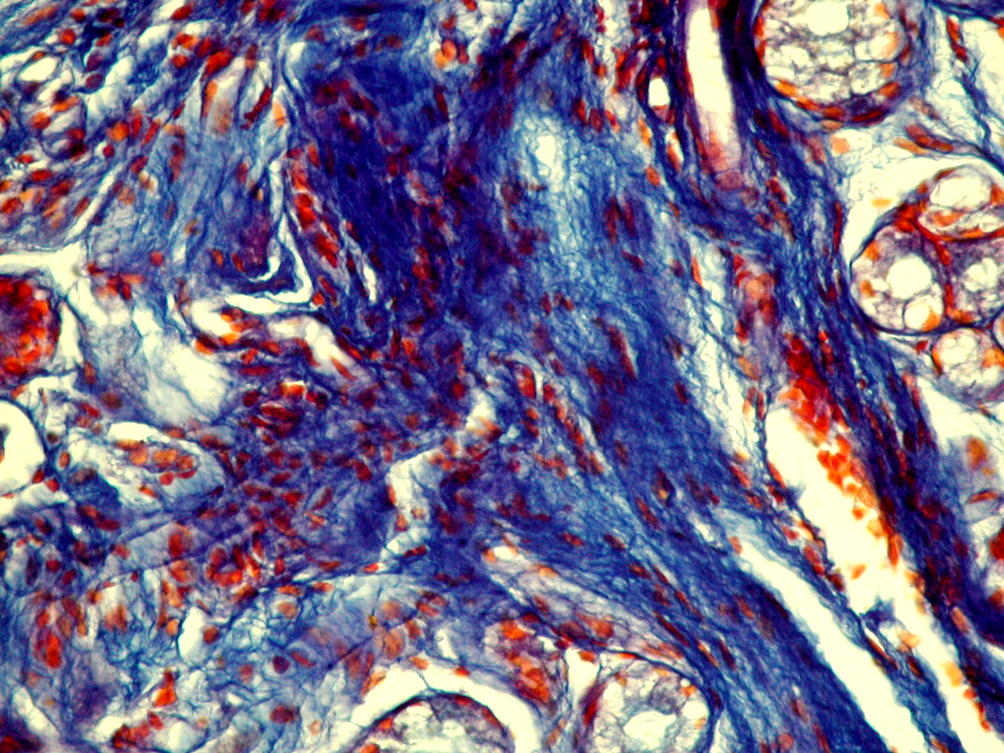
With staining according to Mallory, you can differentiate collagen, elastic and reticular fibers. In the superficial sections, under the subepithelial basement membrane, a dense network of reticular fibers was found, which had a blue color during staining, and their uneven distribution and focal growth were also noted. In the deeper parts of the lamina propria of mucosa, dark blue collagen fibers were found, and their staining was also characterized by unevenness: there were areas of growth of roughened collagen fibers and areas of edema, partially fragmented, without the correct orientation of the fibrils. Elastic red fibers were found in small quantities, which were intertwined between collagen fibers (Fig. 7).
In the comparison group, during Van Gizon staining in the stroma of the own plate were detected fuchsinophilic structures with an ordered parallel orientation. It is known that the phase of fuchsinophilic fibers in this staining corresponds to the advantage of “mature” collagen fibrils. During Mallory staining, fibrous structures were differentiated as collagen, elastic and reticular fibers without signs of their destruction and enhanced growth (Fig. 8).
Therefore, the immunohistochemistry study demonstrated the signs of collagen formation disorders in own plate of the gastric and duodenal mucosa in children and adolescents with gastroduodenal pathology on the background of CTD that were characterized by the weakened synthesis of type III and V interstitial collagens, as well as mature type I collagen deficiency. The non-uniform content of the type IV collagen in basal membranes of epithelium and vessels can be the consequence both of the impaired collagen formation and delayed immune complexes on basal membranes that are not excluded during the inflammatory processes in the mucosa. Considering that the ulcerogenesis is facilitated by changes in the CT state, and the healing quality of the ulcer defect of the gastric and duodenal wall is due to the CT qualitative and quantitative content and synchronization of reparative processes of connective tissue and epithelial elements, it can be assumed that the destructive processes in the gastroduodenal mucosa have been developed on the background of genetic collagen formation disorders, will be severe with long healing periods and frequent regressions.
The obtained data demonstrate a significant effect of the collagen synthesis defects in the development of gastroduodenal pathology in children and adolescents that occurs as the features of morphological changes in the form of synthesis disorder, differentiation, catabolism of the CT fibers structures, and is associated with the reduced functional features. As a result, among children and adolescents with signs of CTD, there is a predominance of lesions of the digestive system, which suggests the need for an in-depth examination of the gastrointestinal tract in patients with suspected dysplasia in order to prevent further development of erosions and ulcers.
Conclusion
- It was found that children with the signs of CTD, such as kyphosis and scoliosis, gothic palate, joint hypermobility, skin fragility and hyperextensibility, mitral valve prolapse, and abnormally placed chords, are more susceptible to the development of pathology of the digestive tract in contrast to their peers with normal connective tissue.
- Gastroduodenal pathology in children and adolescents with CTD is characterized by a combination of the inflammatory process in the mucosa in the different forms of esophagitis and gastritis with frequently impaired motility of the gastroduodenal zone in forms of gastroesophageal and duodenogastric refluxes (77%). The form of mucosal lesions is chronic superficial gastritis with simultaneous inflammation in the antral and fundal parts. Duodenal ulcer was identified rarely (10,0%) and it was accompanied by the detection of Helicobacter pylori.
- Comorbidity of CTD and pathology of the gastrointestinal tract is characterized by the low level of interstitial collagen types I and III in gastric-duodenal mucosa and structural transformation of collagen fibrils (wrong orientation, focal sclerosis, immaturity). It is accompanied by a decrease in mucosa functional ability with the development of the valve-sphincter failure. That is the basis of the formation of motility disorders against the background of insufficiency of the valvar-sphincter apparatus that promotes frequent reflux development.
- The work provides grounds for employing rehabilitation measures among children and adolescents, connected with the early diagnosis of signs of CTD in order to prevent reflux progression and reduce the risk of developing destructive damages and severe chronic diseases (erosion and ulceration) of the digestive system.
Bibliography
- А.G. Kalacheva, O.A. Gromova, N.V. Kerimkulova et al. “Disorders of the formation of connective tissue in children as a consequence of magnesium deficiency”. Attending physician. N. 3 (2012): P. 59.
- Rodney Grahame “Joint hypermobility and genetic collagen disorders: are they related?”, Arch Dis Child (1999); 80: 188–191
- P Tsipouras and F Ramirez. “Genetic disorders of collagen”. J Med Genet. (1987 Jan); 24(1): 2–8. doi: 10.1136/jmg.24.1.2
- Zernova E.S., Kravtsov Yu.A., Yavorskaya M.V. “Peculiaritis of the clinical course of connective tissue dysplasia in children of early age”. Scientific Review. Medical sciences. (2017). No. 4. P.: 21-25; URL: https://science-medicine.ru/ru/article/view?id=1007.
- Grechanina E.Ya., Pesochina E.A., Grechanina Yu.B. “Congenital connective tissue diseases”. Kharkov: Konstanta, (1998). 26 p.
- Demin V.F., Klyuchnikov S.O., Klyuchnikova M.A. “Role of connective tissue dysplasia in the children pathology”. Issues of modern pediatry (2005); 4(1): 50-56.
- Antunes M., Scirè C. A., Talarico R. et al. (2018) “Undifferentiated connective tissue disease: state of the art on clinical practice guidelines”. RMD Open, 4:e000786.doi:10.1136/rmd open-2018-000786.
- Martelli H., Richard S., Moszar M. “Congenital soft tissue dysplasia: a morphological and biochemical study”. Pediatr. Pathol. (1994); 5: 878-894.
- Beighton P., Graham R., Bird H. “Hypermobility of joints”. Heidelberg. New-York, (1983): 178.
- Handler A., Chiild A., Light N.D. “Mitral valve prolapse, aortic compliance, and skin collagen in joint hypermobility syndrome”. Br. Heart J. (1985): 54, 5: 501-508.
- Nica A. E., Alexa L. M., Ionescu A. O. et al. (2016) “Esophageal disorders in mixed connective tissue diseases”. Journal of Medicine and Life., 9 (2), 141-143. PubMed PMID: 27453743; PubMed Central PMCID: PMC4863503.
- Denaxas K., Ladas S. D., Karamanolis G. P. (2018) “Evaluation and management of esophageal manifestations in systemic sclerosis”. Ann. Gastroenterol., 31(2), 165-170.
- P. Weber, G. Ganser, M. Frosch et al. „Twenty-four hour intraesophageal pH monitoring in children and adolescents with scleroderma and mixed connective tissue disease”. J. Rheumatol. (2000), № 27. Р.: 2692–2695.
- I.I. Ivanova, S.F. Gnusaev, Yu.S. Apenchenko et al. “Features of the manifestations of diseases of the digestive tract in children with connective tissue dysplasia”. Questions of modern pediatrics. (2012). T. 11, No.5. P.: 50–55.
- Baia X., Iharaa E, Otsukaa Y. et al. (2019) “Involvement of different receptor subtypes in prostaglandin E2-induced contraction and relaxation in the lower esophageal sphincter and esophageal body”. European Journal of Pharmacology, 857, 172405.
- Kamataki A., Uzuki M., Sawai T. (2015). “Histopathological Change of Esophagus Related to Dysphagia in Mixed Connective Tissue Disease”. Seminars in Dysphagia edited by Renee Speyer and Hans Bogaardt. Chapter 13. http://dx.doi.org/10.5772/60509
- Loeys B.L., Dietz H.C., Braverman A.C., et al. « The revised Ghent nosology for the Marfan syndrome”. Journal of Medical Genetics (2010); 47: 476-485.
- Dixon M., Genta R., Yardley J. “Classification and grading of gastritis”. Am. J. Surg. Pathol. V.20 (1996): 1161 – 1181.
- Brosman M. “Immunofluorescence weathering of formal-paraffin material”. Cs. Patol. (1979); 15, (4): 215-220.
Please check out my research articles here to learn more about my work.




Pingback: (2022) How Do Physical Exercises Affect Learning? Connective Tissue And Hippocampus Secrets | Childhealthcreation.com | How To Grow A Healthy Child?
Pingback: (2022) Connective Tissue And Immune System: Parts Of The Holistic | Childhealthcreation.com | How To Grow A Healthy Child?
Pingback: (2022) Arterial Hypertension In Adolescents - Factors Of Progression And Stabilization | Childhealthcreation.com | How To Grow A Healthy Child?
Pingback: (2022) Main Influencers On The Formation Of A Child's Health | Childhealthcreation.com | How To Grow A Healthy Child?
Pingback: (2022) Evidence-based Approaches To The Prevention Of Gastrointestinal Pathology In Children And Adolescents With Non-inflammatory Hereditary Connective Tissue Disorders | Childhealthcreation.com | How To Grow A Healthy Child?